Atomic Bonding
-
The mechanisms of bonding between the atoms are based on the foregoing discussion on electrostatic interatomic interaction.
-
The types of bond and bond strength are determined by the electronic structures of the atoms involved.
-
The valence electrons take part in bonding. The atoms involved acquire, loose or share valence electrons to achieve the lowest energy or stable configuration of noble gases.
- primary bonding
- secondary bonding
Atomic bonding can be broadly classified as
Atomic Bonding Primary Bonds
Three types primary bonds are found in solids
- Ionic
- Covalent
- Metallic
Majority of the engineering materials consist of one of these bonds. Many properties of the materials depend on the specific kind of bond and the bond energy.
1.Ionic Bond
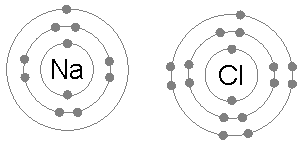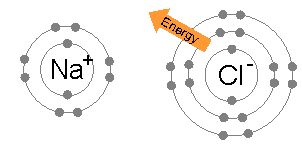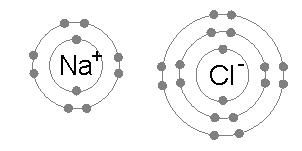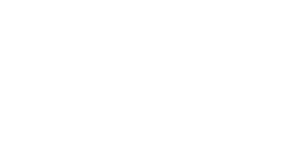
Ionic bonds are generally found in compounds composed of metal and non-metal and arise out of electrostatic attraction between oppositely charged atoms (ions).
Number of electron in outer shell is 1 in Na and 7 in Cl . Therefore, Na will tend to reject one electron to get stable configuration of Ne and Cl will accept one electron to obtain Ar configuration. The columbic attraction between Na+ and Cl¯ions thus formed will make an ionic bond to produce NaCl. Some other examples are CaF2, CsCl , MgO, Al2O3.
2.Covalent Bond
In this type of bonding, atoms share their valence electrons to get a stable configuration.
Methane (CH4):
Four hydrogen atoms share their valence electrons with one carbon atom and the carbon atom in turn shares one valence electron with each of the four hydrogen atoms. In the process both H and C atoms get stable configuration and form a covalent bond.
- Covalent bonds are formed between atoms of similar electronegativity.
-
Carbon atoms in diamond are covalently bonded to each other.
-
Silicon also has valency of four and forms SiC through covalent bonding with C atoms
Metallic Bond
In metals the valence electrons are not really bound to one particular atom, instead they form a sea or cloud of valence electrons which are shared by all the atoms. The remaining electrons and the nuclei form what is called the ion core which is positively charged.
The metallic bond arises out of the columbic attraction between these two oppositely charged species – the electron cloud and the ion cores
Characteristics of primary bonds
Structure-property correlation
-
Ionic and covalent bonds posses high bond energy – 450 – 1000 kJ/mole
- High bond strength in ionic and covalent solids results in high melting point, high strength and hardness. e.g. diamond
- As the electrons are tightly bound to the atoms they are generally poor conductors of heat and electricity
- Are brittle in nature
- Most of the ceramics consist of covalent (SiC) or ionic bonds (Al2O3) or a mix of both and hence, exhibit all the properties described above.
Structure-property correlation
-
Metallic bonds on the other hand provide good thermal and electrical conductivities as the valence electrons are free to move.
- The metallic bond energy is 68 kJ/mol (Hg) on the lower side and 850 kJ/mol (W, tungsten) on the higher side.
- Bond strength increases with atomic number as more electrons are available to form the bonds with the ion cores. As a result melting point, hardness and strength increases with atomic number.
- Metals are ductile as the free moving electrons provides agility
Secondary Bonds
Secondy bonds can be classified as
- Wander wall bonding
- Secondry bonding
Van der Waals bonding
-
Van der Waals bonding between molecules or atoms arise due to weak attraction forces between dipoles
- The natural oscillation of atoms leading to momentary break down of charge symmetry can generate temporary dipoles
- Dipoles can induce dipoles and attraction between opposites ends of the dipoles leads to weak bonding
Van der Waals Bonding
- An ion can also induce a dipole
-
Some molecules like HCl have permanent dipoles due to asymmetrical arrangement of +ve and –ve charges.
-
Van der Waals bonding is much weaker compared to primary bonds. Bond energy lies in the range of 2 – 10 kJ/mol.
-
Molecules in liquid and gas are held by weak Van der Waals forces
Van der Waals bonds
-
The atomic layers in graphite are held together by weak van der Waals bonds. Therefore, the layers can move easily over each other and this imparts the lubricating property graphite is known for.
Hydrogen bonding
- Hydrogen bond is a type of secondary bond found in molecules containing hydrogen as a constituent.
-
The bond originates from electrostatic interaction between hydrogen and another atom of high electronegativity such as fluorine or oxygen.
- The strength of hydrogen bonds is in the range of 10 - 50 kJ/mol.
-
Water molecules, for example, are connected by hydrogen bonds (dashed lines in the picture).
H2O
Evaluation and Examples At this point one should be able to
-
Understand two quantum mechanics models of atomic structure and their fundamental differences. Understand quantum numbers and their significance.
- Find out electronic configuration of a given element.
Understand atomic interactions and different types of atomic bonding.
- Explain some properties based on atomic bonding
Solid Solution
- Alloy
Alloy is a metal, composing of a mixture of elements. Most of alloys are composed of a base metal with small amounts of additives or alloying elements. The typical examples of alloys are steel/cast iron (iron base alloys), bronze/brass (copper base alloys), aluminum alloys, nickel base alloys, magnesium base alloys, titanium alloys.
- When two metals are mixed together they form an alloy if one metal is soluble in the other one in solid state. Therefore, an alloy is a solid solution of two or more metals.
- Primarily there are two types of solid solutions
- Substitutional –
Solute atoms occupy the regular lattice sites of the parent metal (solvent). Substitutional solid solutions can be random (Cu-Ni) or ordered (Cu-Au).
- Interstitial –
Solute atoms occupy the interstitial positions (Steel – C solute atoms in Fe) .
Solvent Solute
Random substitutional Ordered substitutional Interstitial
Hume-Rothery Rules
Formation of substitutional solid solutions between two metals is governed by a set of rules known as Hume-Rothery rules
-
Size difference between the atoms of solute and the parent metal should be less than 15%.
- The electronegetivity difference between the metals should be small (minimum chemical affinity to each other).
- The solubility of a metal with higher valence in a solvent of lower valence is more compared to the reverse situation e.g. Zn is much more soluble in Cu than Cu in Zn.
- For complete solubility over the entire range of compositions the crystal structures of the solute and the solvent must be the same.
Ordering in Solid solutions
As stated before substitutional solid solutions can be either ordered or random. This depends on a thermodynamic parameter called enthalpy of mixing, Hmix Gmix = Hmix -T Smix
Gmix is the Gibbs free energy change and Smix entropy of mixing.
For an ideal solution
Hmix = 0. If Hmix> 0,
formation of like bonds (A-A or B-B) is preferred in a solid solution between metals A and B. This known as clustering.
If Hmix< 0, unlike bonds (A-B) are preferred. This leads to ordering which may exist over short range or long range (at lower temperatures).
Intermediate Phases
As the name suggest intermediate structures formed between two metals are neither the parent metals nor like an alloy.
Intermetallic compounds :
If two elements have high difference in electronegetivity, they tend to from a system called intermetallic compound. Intermetallic compounds like MgSe, PbSe, Mg2Si, Cu2S are cubic whereas NiAs, MnSe, CuSn are hexagonal.
Electron or Hume Rothery phases
These compounds have wide range of solubility and occur at certain values of valence electrons to atom ratio such as 3:2 (CuZn), 21:13 (Cu5Zn8), 7:4 (CuZn3).
Laves phase
Laves phases have a general formula of AB2, for example MgCu2 (cubic), MgZn2 (hexagonal), MgNi2 (hexagonal)
Sigma phase
Sigma phase has a very complex crystal structure and is very brittle. This phase can act as a source of embrittlement in some alloys such as steels.
Metal carbides and nitrides
Metals which have high chemical affinity for carbon and nitrogen form carbides and nitrides such as VC, NbC, VN, NbN, TiC, TiN. They can act as source of hardening in many alloys.
- Ionic
- Covalent
- Metallic
Methane (CH4):
Four hydrogen atoms share their valence electrons with one carbon atom and the carbon atom in turn shares one valence electron with each of the four hydrogen atoms. In the process both H and C atoms get stable configuration and form a covalent bond.
- Covalent bonds are formed between atoms of similar electronegativity.
- Carbon atoms in diamond are covalently bonded to each other.
- Silicon also has valency of four and forms SiC through covalent bonding with C atoms
- Ionic and covalent bonds posses high bond energy – 450 – 1000 kJ/mole
- High bond strength in ionic and covalent solids results in high melting point, high strength and hardness. e.g. diamond
- As the electrons are tightly bound to the atoms they are generally poor conductors of heat and electricity
- Are brittle in nature
- Most of the ceramics consist of covalent (SiC) or ionic bonds (Al2O3) or a mix of both and hence, exhibit all the properties described above.
- Metallic bonds on the other hand provide good thermal and electrical conductivities as the valence electrons are free to move.
- The metallic bond energy is 68 kJ/mol (Hg) on the lower side and 850 kJ/mol (W, tungsten) on the higher side.
- Bond strength increases with atomic number as more electrons are available to form the bonds with the ion cores. As a result melting point, hardness and strength increases with atomic number.
- Metals are ductile as the free moving electrons provides agility
Secondy bonds can be classified as
- Van der Waals bonding between molecules or atoms arise due to weak attraction forces between dipoles
- The natural oscillation of atoms leading to momentary break down of charge symmetry can generate temporary dipoles
- Dipoles can induce dipoles and attraction between opposites ends of the dipoles leads to weak bonding
- An ion can also induce a dipole
- Some molecules like HCl have permanent dipoles due to asymmetrical arrangement of +ve and –ve charges.
- Van der Waals bonding is much weaker compared to primary bonds. Bond energy lies in the range of 2 – 10 kJ/mol.
- Molecules in liquid and gas are held by weak Van der Waals forces
- The atomic layers in graphite are held together by weak van der Waals bonds. Therefore, the layers can move easily over each other and this imparts the lubricating property graphite is known for.
- Hydrogen bond is a type of secondary bond found in molecules containing hydrogen as a constituent.
- The bond originates from electrostatic interaction between hydrogen and another atom of high electronegativity such as fluorine or oxygen.
- The strength of hydrogen bonds is in the range of 10 - 50 kJ/mol.
- Water molecules, for example, are connected by hydrogen bonds (dashed lines in the picture). H2O
- Understand two quantum mechanics models of atomic structure and their fundamental differences. Understand quantum numbers and their significance.
- Find out electronic configuration of a given element. Understand atomic interactions and different types of atomic bonding.
- Explain some properties based on atomic bonding
- Alloy
Alloy is a metal, composing of a mixture of elements. Most of alloys are composed of a base metal with small amounts of additives or alloying elements. The typical examples of alloys are steel/cast iron (iron base alloys), bronze/brass (copper base alloys), aluminum alloys, nickel base alloys, magnesium base alloys, titanium alloys. - When two metals are mixed together they form an alloy if one metal is soluble in the other one in solid state. Therefore, an alloy is a solid solution of two or more metals.
- Primarily there are two types of solid solutions
- Substitutional – Solute atoms occupy the regular lattice sites of the parent metal (solvent). Substitutional solid solutions can be random (Cu-Ni) or ordered (Cu-Au).
- Interstitial – Solute atoms occupy the interstitial positions (Steel – C solute atoms in Fe) . Solvent Solute Random substitutional Ordered substitutional Interstitial
- Size difference between the atoms of solute and the parent metal should be less than 15%.
- The electronegetivity difference between the metals should be small (minimum chemical affinity to each other).
- The solubility of a metal with higher valence in a solvent of lower valence is more compared to the reverse situation e.g. Zn is much more soluble in Cu than Cu in Zn.
- For complete solubility over the entire range of compositions the crystal structures of the solute and the solvent must be the same.
As stated before substitutional solid solutions can be either ordered or random. This depends on a thermodynamic parameter called enthalpy of mixing, Hmix Gmix = Hmix -T Smix
Gmix is the Gibbs free energy change and Smix entropy of mixing.
Gmix is the Gibbs free energy change and Smix entropy of mixing.








Comments
Post a Comment